What is the law of conservation of charge
The concept of "electric charge»First introduced in 1875 in this. Wording pendant law claims that the force that acts between two charged particles directed in a straight line is directly proportional to the charge and inversely proportional to the square of the distance between them.
This means that, having removed the charges by 2 times, the force of their interaction will decrease by four times. And this is how it looks in vector form:
The limit of applicability of the above:
- point charges;
- evenly charged bodies;
- its effect is valid at large and small distances.
The merits of Charles Coulomb in the development of modern electrical engineering are great, but let's move on to the main topic of the article - the law of conservation of charge. He claims that the sum of all charged particles in a closed system is unchanged. In simple words, charges cannot arise or disappear just like that. Moreover, it does not change in time and can be measured (or divided, quantized) by parts that are multiples of an elementary electric charge, that is, an electron.
But remember that in an isolated system, new charged particles arise only under the influence of certain forces or as a result of any processes. So ions arise as a result of gas ionization, for example.
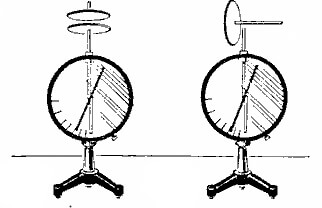
If you are concerned about the question, by whom and when is the law of conservation of charge open? It was confirmed in 1843 by the great scientist - Michael Faraday. In experiments confirming the conservation law, the number of charges is measured by electrometers, its appearance is shown in the figure below:
But we will confirm our words with practice. Take two electrometers, put a metal disk on the rod of one, cover it with cloth. Now we need another metal disk on the dielectric handle. His three are on a disk lying on an electrometer, and they are electrified. When the disk with the dielectric handle is removed - the electrometer will show how charged it has become, with the disk with the dielectric handle we touch the second electrometer. His arrow will also deviate. If now we close two electrometers with a rod on dielectric handles - their arrows return to their original position. This suggests that the total or resulting electric charge is zero, and its value in the system remains the same.
From here follows the formula describing the law of conservation of electric charge:
The following formula suggests that a change in the electric charge in the volume is equivalent to the total current through the surface. This is also called the "continuity equation."
And if we pass to a very small volume, we get the law of conservation of charge in a differential form.
It is also important to tell how the charge and mass number are related. When talking about the structure of substances, such words as molecules, atoms, protons and the like often sound. So the mass number is the total number of protons and neutrons, and the number of protons and electrons in the nucleus is called the charge number. In other words, the charge number is the charge of the nucleus, and it always depends on its composition. Well, the mass of an element depends on the number of its particles.
In the end, we recommend watching a video on which this whole topic is examined in more detail:
Thus, we briefly examined issues related to the law of conservation of electric charge. It is one of the fundamental laws of physics along with the laws of conservation of momentum and energy. Its action is impeccable and over time and the development of technology fails to refute its justice. We hope that after reading our explanation you will understand all the key points of this law!
Related materials:

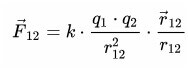

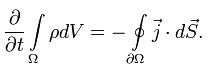
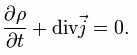




Good day! What does an electric body mean? Thanks in advance. Thank.
Hello! Probably you mean an electrically charged body. In the case when the body is electrically neutral - the number of negative charges is balanced by the number of positive charges, if the balance is broken - the body becomes electrically charged negatively or positively.